

That prompted the FDA and Pfizer-BioNTech to hold off on reviewing that data until the companies provided additional information on whether adding a third dose would increase levels of virus-fighting antibodies, and therefore immunity, against COVID-19. But those two doses generated relatively low levels of immunity, in part because they were tested while Omicron was dominating the U.S.
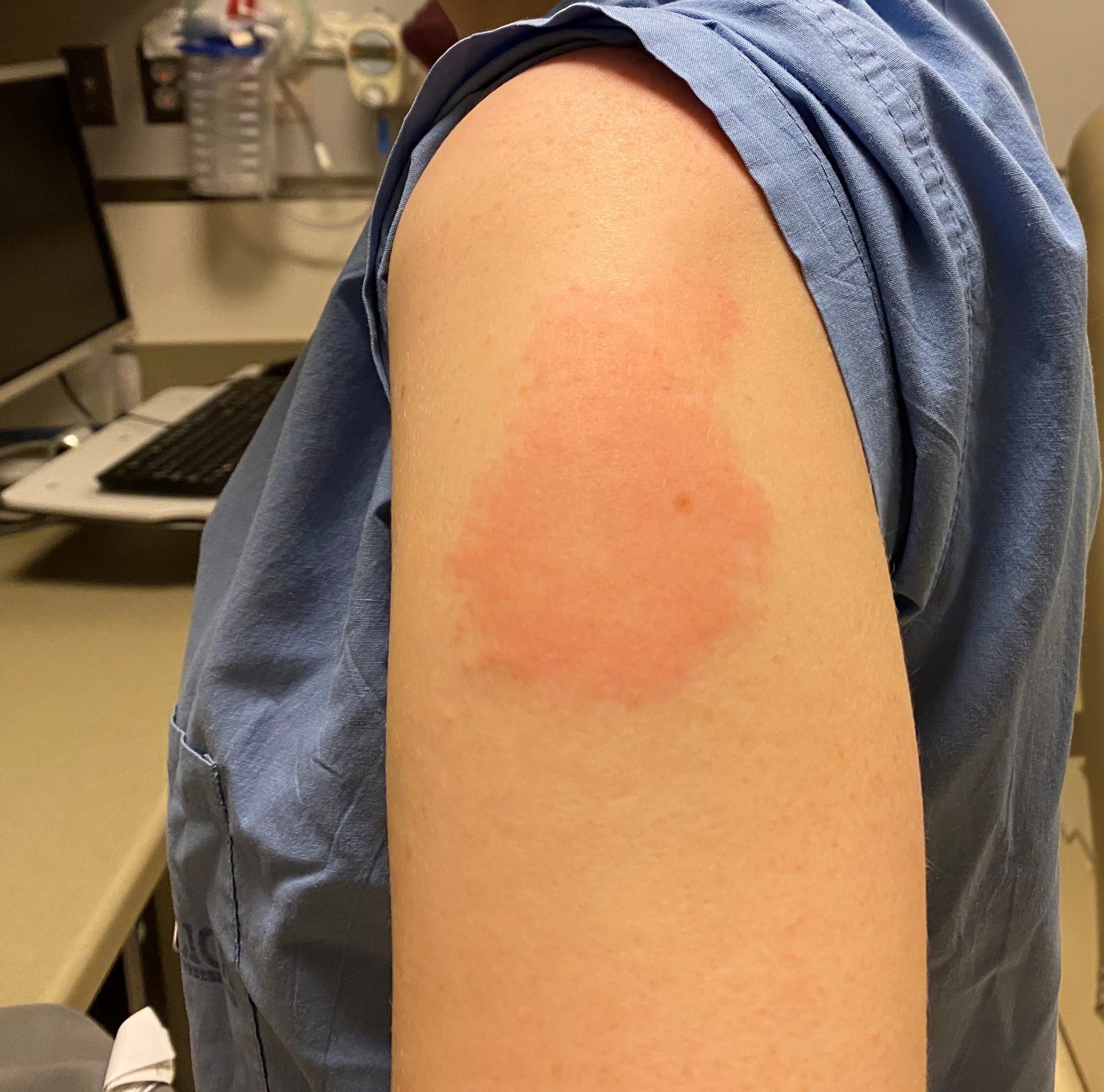
The companies began submitting their vaccine for FDA authorization in this age group last December, after gathering data on a two-dose course. It’s been a long road for the Pfizer-BioNTech vaccine. In the pediatric group, three-dose vaccine efficacy against symptomatic disease was 80.4% during a time when Omicron was circulating widely in the U.S.-though that estimate was based on such a small number of cases that it’s difficult to draw firm conclusions about how protective it is in real-world situations. This allowed researchers to infer how much those antibodies protected against COVID-19. In a company study involving more than 4,500 children, antibody levels generated by these children were compared to those produced by vaccinated people ages 16 to 25 years old. Children would receive two doses three weeks apart, and a third dose at least two months later. COVID-19 vs.The Pfizer-BioNTech vaccine for kids ages 6 months to 4 years old is a three-shot regimen, and each dose is one-tenth the dosage given to adults.COVID-19 vaccines for kids: What you need to know.COVID-19 vaccine: Should I reschedule my mammogram?.COVID-19 drugs: Are there any that work?.

MMWR Morbidity and Mortality Weekly Report. Post-COVID conditions among adult COVID-19 survivors aged 18-64 and ≥ 65 years - United States, March 2020 - November 2021. Patient tips: Healthcare provider appointments for post-COVID conditions.Centers for Disease Control and Prevention. Multisystem inflammatory syndrome (MIS).Coronavirus disease 2019 and cardiovascular complications: Focused clinical review. COVID-19: Evaluation and management of adults following acute viral illness. Post-COVID conditions: Overview for healthcare providers.Chronic fatigue syndrome involves extreme fatigue that worsens with physical or mental activity, but doesn't improve with rest. Some symptoms are similar to those caused by chronic fatigue syndrome and other chronic illnesses that develop after infections.
It's also not clear if post- COVID-19 syndrome is new and unique to COVID-19. Keep in mind that it can be hard to tell if you are having symptoms due to COVID-19 or another cause, such as a preexisting medical condition.


 0 kommentar(er)
0 kommentar(er)
